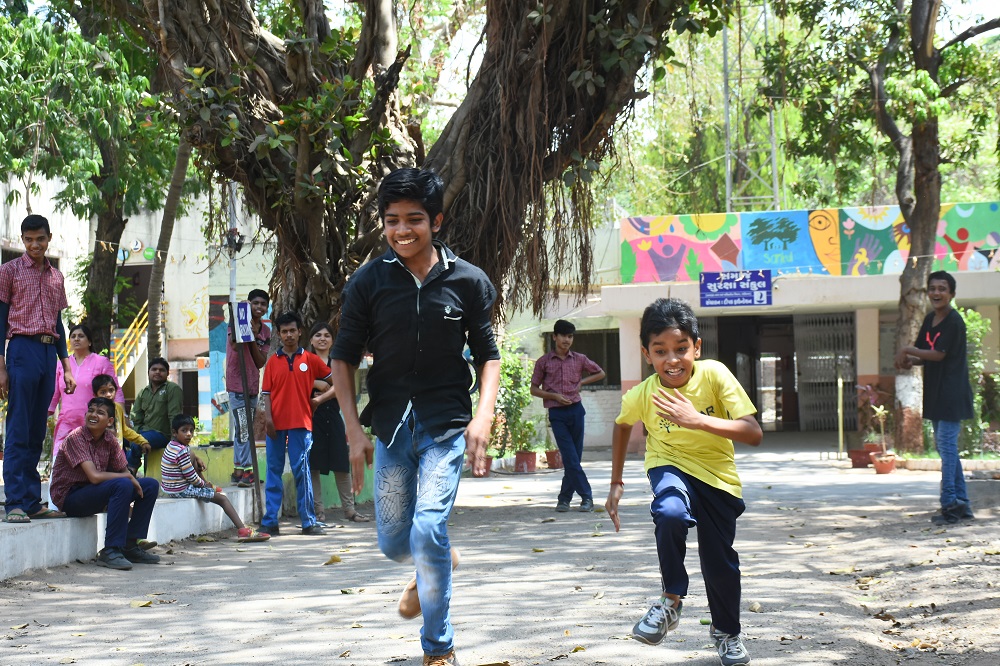India has a coastline of 11098.81 GOI 29th April 2025 (old 7516 km) and Gujarat has the biggest coastline in the country with 2340.62 km (old 1600km) followed by Maharashtra with 877.97km. The coastline of Gujarat has two Gulfs; the Gulf of Kutch and the Gulf of Khambhat.
The coasts are home to unique ecosystems like mangroves, coral reefs and estuaries. These ecosystems are crucial for biodiversity and provide natural protection against erosion and extreme weather events besides providing critically important minerals for various industrial needs.
The State of Forest Report 2023 has reported the total Mangrove cover of the country as 4,991.68 sq. km, 0.15 % of total geographical area along the 11098.81 km long Indian coastline, with Very Dense Mangrove – 1,463.97 sq. km (29.33%), Moderately Dense Mangrove – 1,500.84 km2 (30.07%), Open Mangroves constitute – 2,026.87 sq. km (40.60%). In comparison to the 2021 assessment, there is a net decrease of 7.43 sq. km in the country’s Mangrove coverage, with Gujarat having a notable decrease of 36.39 sq. km.
Due to rampant human exploitation, urban development, and climate change impacts leading to coastal erosions the mangrove cover is declining. Mangroves are not only ecological powerhouses but also guardians of coastal resilience, protecting vulnerable communities from climate-induced sea-level rise and extreme weather events. The Gulf of Khambhat is oriented with its tail towards the north and mouth towards the south covering about 3,120 sq. km. The funnel like structure of the gulf with wide mouth and narrow head (width 200 km at mouth of Gulf terminating to ~6 km at the tail) makes the Gulf second in the world in terms of tidal amplitude. Several rivers including major rivers of Gujarat like Narmada, Tapi, Sabarmati and Mahi open in the Gulf, draining down water and alluvium to the gulf and coastal area.
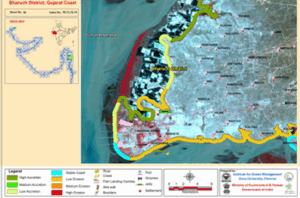
The coastline of Bharuch situated along the Gulf of Khambhat near Dahej is highly prone to erosion according to study on the status of Shoreline Changes along the Gujarat Coast (map.1). Deepak Foundation has initiated a three-pronged approach for the protection of the coastline along Paniyadra village in Bharuch district: Habitat restoration, Biodiversity documentation and Community sensitization.

Eroded coastline near Jageshwar village- Dahej.
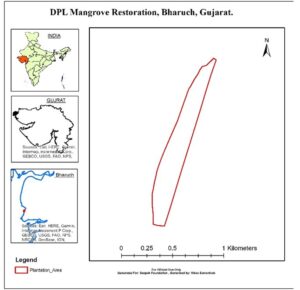
Deepak Foundation with support from Deepak Phenolics Limited has initiated Mangrove restoration along the high erosion zone near Paniyadra village under Vagra block and expanding in Jhambusar block of Bharuch district. An area of 100 acres have been identified for the restoration in cooperation with the local community and the state forest department (map).
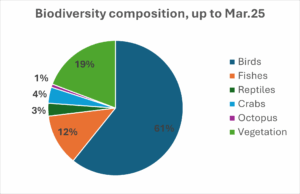
Along with the Avicennia marina plantation in 100 acres we are creating a database of the biodiversity found in its vicinity and till March 2025 our team has documented more than 130 biodiversity species including plants, fishes, reptiles, crabs and birds in Vagra block, which is 3% of total Mangrove Forest biodiversity of India. An earlier study by GEC in 2011 had documented 44 biodiversity species from Vagra block, an increase of 195% in 13 years period but as the study is ongoing, we expect it will increase further.
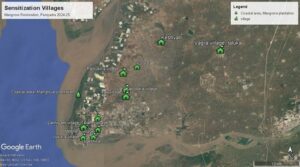
To sensitise the community and students we have developed outreach materials outlining the role and importance of mangroves for humans and the associated wildlife. Through 26 outreach programs in 12 villages along the coastal area we have reached out to 1829 people in the area and expanding further.
Outreach programs in villages.


Common Pleco, Hypostomus plecostomus

Puffer, Takifugu oblongus

Old lady Octopus Cistopus indicus

Chiloscyllium arabicum (Near Threatened)






























 Nidaan Van will have been acknowledged to be useful in providing quality diagnostic services for underprivileged and hard to reach areas where diagnostic facilities/providers are non-existent, non-functional, or inaccessible.
Nidaan Van will have been acknowledged to be useful in providing quality diagnostic services for underprivileged and hard to reach areas where diagnostic facilities/providers are non-existent, non-functional, or inaccessible.



 Deepak Foundation has significantly contributed to women’s empowerment by giving them opportunities to enhance their livelihood. Women in Nandesari, a small, closed community, now have the opportunity to gain new skills and ensure a living thanks in large part to campaigns that support women’s self-help groups (SHGs) and women’s dairy cooperatives (WDCs).
Deepak Foundation has significantly contributed to women’s empowerment by giving them opportunities to enhance their livelihood. Women in Nandesari, a small, closed community, now have the opportunity to gain new skills and ensure a living thanks in large part to campaigns that support women’s self-help groups (SHGs) and women’s dairy cooperatives (WDCs).